-
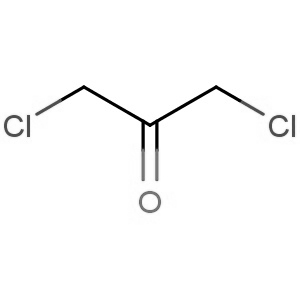 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
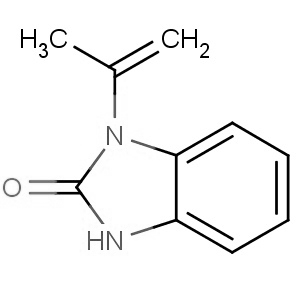 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
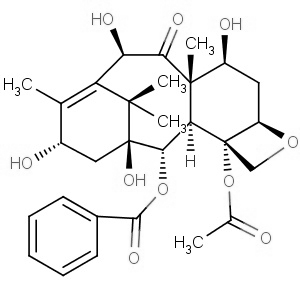 We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products
We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products -
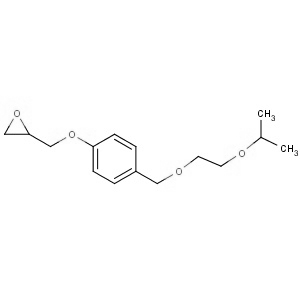
We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality.
Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products
-

We offer this product from a manufacturer in India led by seasoned, experienced techno-commercial professionals in Ahmadabad, Gujarat (India). With an experienced team of scientists & engineers; GLP compliant labs, and WHO GMP manufacturing plants in Ahmedabad, the factory is suitably placed to quickly supply quality products APIs and Intermediates across the scale and solve our clients? challenging development, scale-up & regulatory needs by offering a broad range of development services.
-

We offer this product from a manufacturer in India led by seasoned, experienced techno-commercial professionals in Ahmadabad, Gujarat (India). With an experienced team of scientists & engineers; GLP compliant labs, and WHO GMP manufacturing plants in Ahmedabad, the factory is suitably placed to quickly supply quality products APIs and Intermediates across the scale and solve our clients? challenging development, scale-up & regulatory needs by offering a broad range of development services.
-
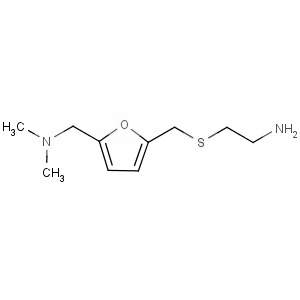 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
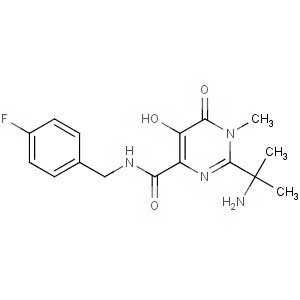
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-morpholinyl)phenyl]amino]propyl]-1H-isoindole-1,3(2H)-dione](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/446292-07-5.jpg)
![2-[[(5S)-2-Oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-1H-isoindole-1,3(2H)-dione](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/446292-08-6.jpg)