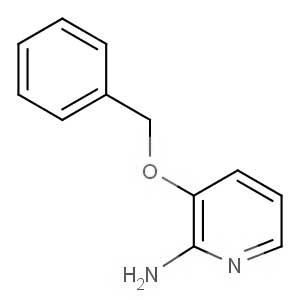
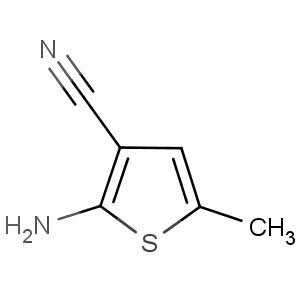
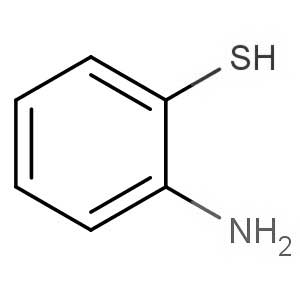
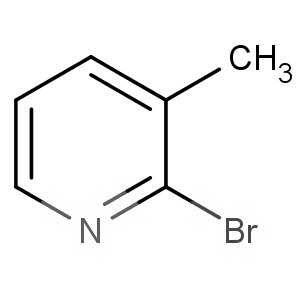
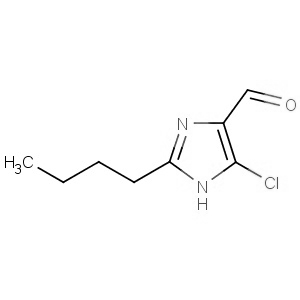
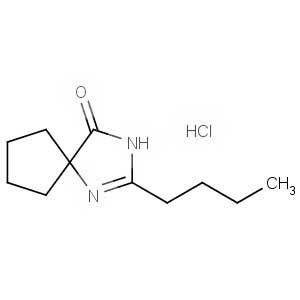
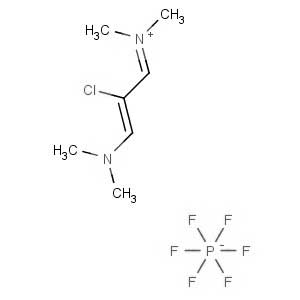
We offer this product from an Indian manufacturer focused on developing and manufacturing of Active Pharmaceutical Ingredients (APIs), Pharma Intermediates, Fine Chemicals and Pyridine Derivatives in a cGMP factory with ISO 9001-2008 and ISO 14000:2004 certification.
-
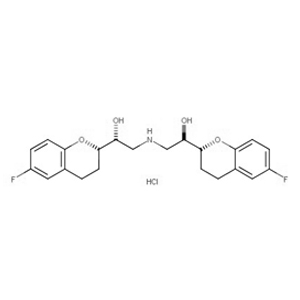 We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage. Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.
We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage. Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.
![2-Acetylbenzo[b]thiophene](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/22720-75-8.jpg)