We offer this product from a manufacturer in CHINA mainly engaged in R&D and production of pharmaceutical intermediates. Quality management systems are strictly under the supervision of ISO9001 and ISO14001 system.
-
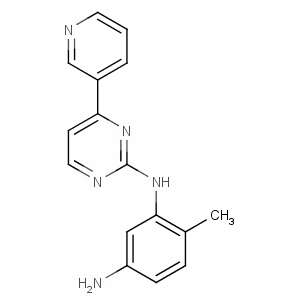 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
![N-[(S)-1-Carbethoxy-1-butyl]-(S)-alanine](https://manusakttevabiopharma.com/wp-content/uploads/2021/12/82834-12-6.jpg)
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
-
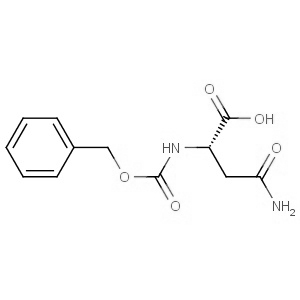 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
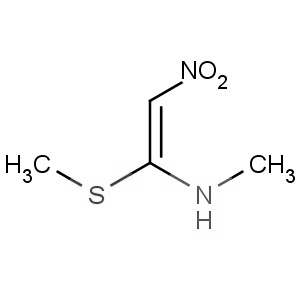 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
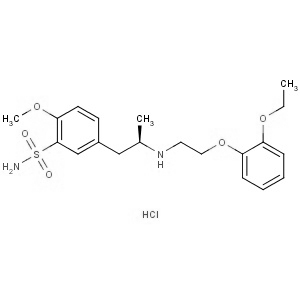 We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage. Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.
We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage. Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.

![N-[(2Z)-2-Chloro-3-(dimethylamino)-2-propenylidene]-N-methylmethanaminium hexafluorophosphate](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/291756-76-8.jpg)
![N-[(4-Aminophenyl)iminomethyl]carbamic acid hexyl ester hydrochloride](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/1307233-93-7.jpg)
![N-[[2-(Chloromethyl)-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-beta-alanine ethyl ester](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/1307233-94-8.jpg)
![N-[2-[4-[N-(Hexyloxycarbonyl)amidino]phenylaminomethyl]-1-methyl-1H-benzimidazol-5-ylcarbonyl]-N-(2-pyridyl)-beta-alanine ethyl ester](https://manusakttevabiopharma.com/wp-content/uploads/2022/01/211915-06-9.jpg)