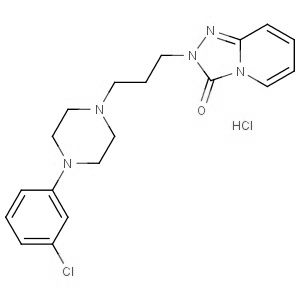
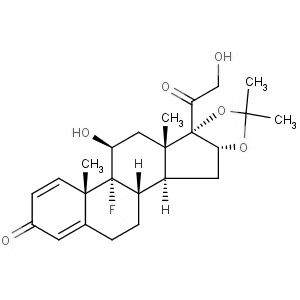
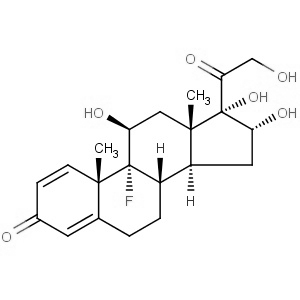
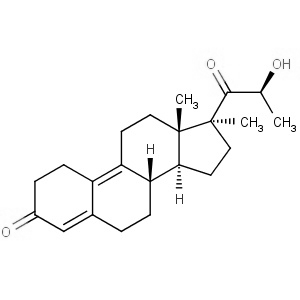
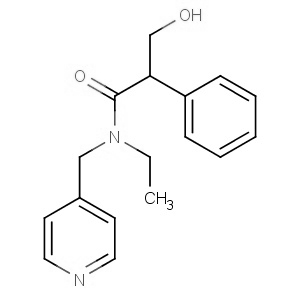
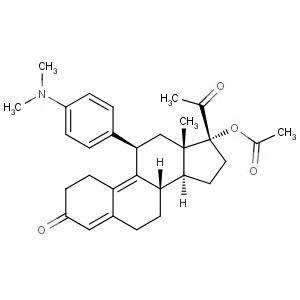
We offer this product from a manufacturer in India having ISO 9001: 2008, ISO 14001, ISO 18001 and are a WHO - GMP Certified Company.
As a Pharmaceutical company, the factory manufactures all the APIs developed in their own R&D. Currently, and the R&D is involved in developing a few more API products. We have a Biotech drug discovery R&D jointly developed with an Israeli partner to produce Recombinant fusion proteins to treat and cure asthma and Mastocytosis.