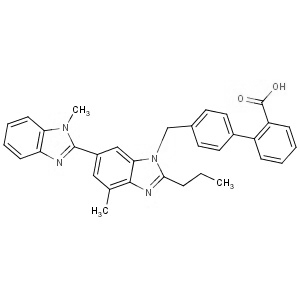
-
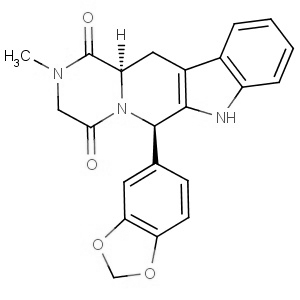 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -

We offer this product from a manufacturer in India having ISO 9001: 2008, ISO 14001, ISO 18001 and are a WHO - GMP Certified Company.
As a Pharmaceutical company, the factory manufactures all the APIs developed in their own R&D. Currently, and the R&D is involved in developing a few more API products. We have a Biotech drug discovery R&D jointly developed with an Israeli partner to produce Recombinant fusion proteins to treat and cure asthma and Mastocytosis.
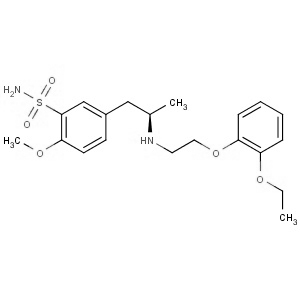
-
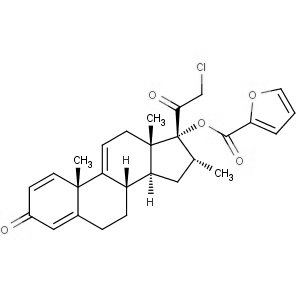
 We offer this product from an Indian manufacturer having two-world class manufacturing sites, USFDA and COFEPRIS approved, WHO GMP certified, and have received AFM certificate from Japanese PMDA and recently concluded Korean FDA audit successfully. Further, the facility is also compliant with all other major regulatory bodies, including UKMHRA, EDQM, and TGA.
We offer this product from an Indian manufacturer having two-world class manufacturing sites, USFDA and COFEPRIS approved, WHO GMP certified, and have received AFM certificate from Japanese PMDA and recently concluded Korean FDA audit successfully. Further, the facility is also compliant with all other major regulatory bodies, including UKMHRA, EDQM, and TGA. -
We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage.
Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.
-

We offer this product from a manufacturer in India who specializes in exploring the untapped potential for Drug Intermediates. With the customer base expanding manifold and demanding more products, the unit grew further with the focus on APIs and the Intermediates. This plant has been designed keeping in view cGMP requirements & 21 CFR norms.
-

We offer this product from a manufacturer in India having a global manufacturing footprint that helps us deliver quality-assured products to our customers worldwide. In line with the growth strategy, we continue to ramp up capacities and automation to support the pipeline of complex generics and biotechnology products.
-
 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -

We offer this product from a manufacturer in India having a global manufacturing footprint that helps us deliver quality-assured products to our customers worldwide. In line with the growth strategy, we continue to ramp up capacities and automation to support the pipeline of complex generics and biotechnology products.