We offer this product from a manufacturer in India specializing in Prescription drugs, Generic, OTC Products, Intravenous infusion, and Bulk Actives with 25years of experience. Both APIs and FDF?s are being manufactured at world-class manufacturing facilities.
-
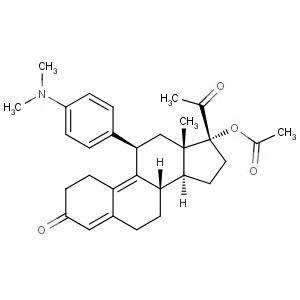
We offer this product from the API manufacturing facility located in India consisting of different volumes of reactors suitable to carry the reactions like Catalytic hydrogenations, Chlorination?s, Nitration?s, Alkylation?s, Oxidations, Reductions, Resolutions(chemical), Couplings, etc. The manufacturing unit has a well-equipped ceanroom facility for finished product drying, milling, packing & storage.
Quality Control is independent of production and is done in-house at the on-site QC laboratory, including GC & HPLC. Documentations are prepared and upgraded as per GMP guidelines like ICH Q7 and other international regulatory guidelines. Regular Internal Quality Audits and GMP Audits to ensure the continuous maintenance of Change control, Document control, Deviation Handling, Complaint handling, Validation Qualification of Equipment, and total Quality system. The unit has an excellent laboratory to conduct experiments on existing products and other new molecules development.
-

We offer this product from a manufacturer in India having ISO 9001: 2008, ISO 14001, ISO 18001 and are a WHO - GMP Certified Company.
As a Pharmaceutical company, the factory manufactures all the APIs developed in their own R&D. Currently, and the R&D is involved in developing a few more API products. We have a Biotech drug discovery R&D jointly developed with an Israeli partner to produce Recombinant fusion proteins to treat and cure asthma and Mastocytosis.
-
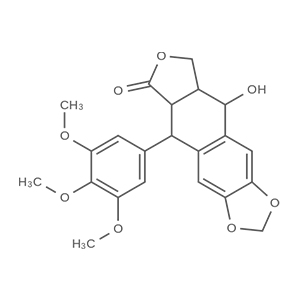 We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products
We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products -
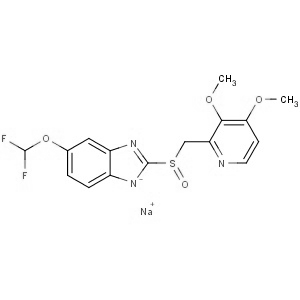 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
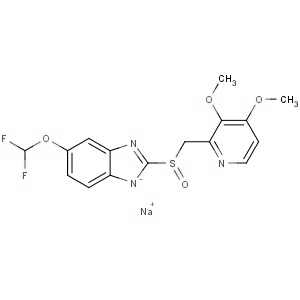
We offer this product from an Indian manufacturer having two-world class manufacturing sites, USFDA and COFEPRIS approved, WHO GMP certified, and have received AFM certificate from Japanese PMDA and recently concluded Korean FDA audit successfully. Further, the facility is also compliant with all other major regulatory bodies, including UKMHRA, EDQM, and TGA.
-
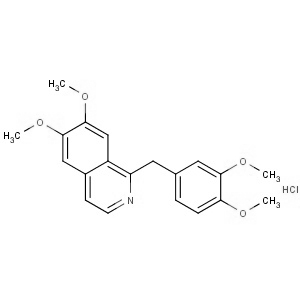 We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products
We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality. Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products -

We offer this product from a manufacturer in India having ISO 9001: 2008, ISO 14001, ISO 18001 and are a WHO - GMP Certified Company.
As a Pharmaceutical company, the factory manufactures all the APIs developed in their own R&D. Currently, and the R&D is involved in developing a few more API products. We have a Biotech drug discovery R&D jointly developed with an Israeli partner to produce Recombinant fusion proteins to treat and cure asthma and Mastocytosis.