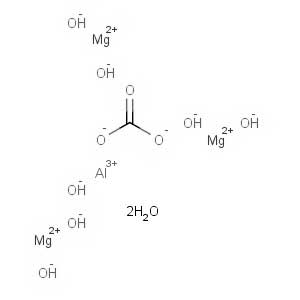
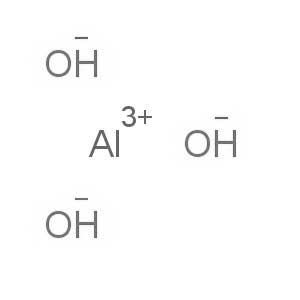
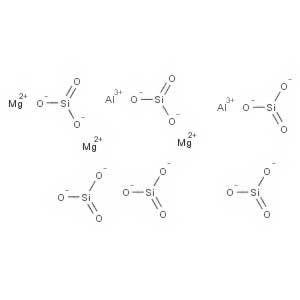
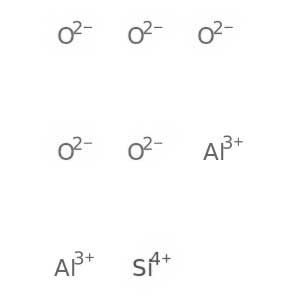
-
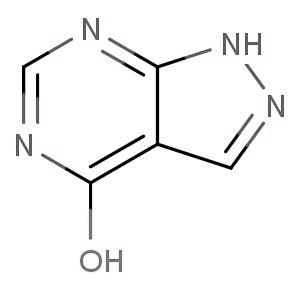 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
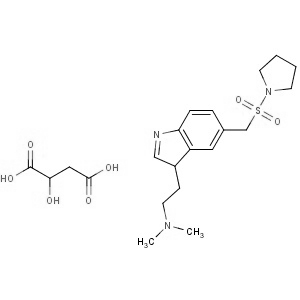 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
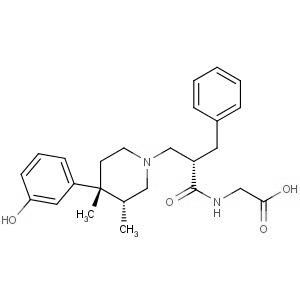 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -
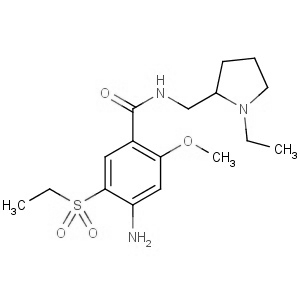 We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
We are offering the product from our Indian manufacturer's USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India). -

We offer this product from a manufacturer in India having ISO 9001: 2008, ISO 14001, ISO 18001 and are a WHO - GMP Certified Company.
As a Pharmaceutical company, the factory manufactures all the APIs developed in their own R&D. Currently, and the R&D is involved in developing a few more API products. We have a Biotech drug discovery R&D jointly developed with an Israeli partner to produce Recombinant fusion proteins to treat and cure asthma and Mastocytosis.