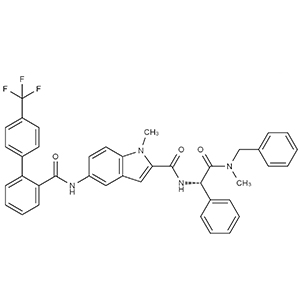
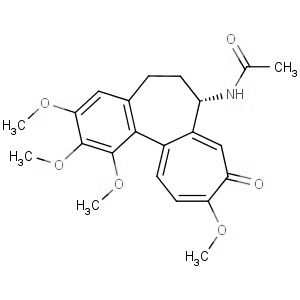
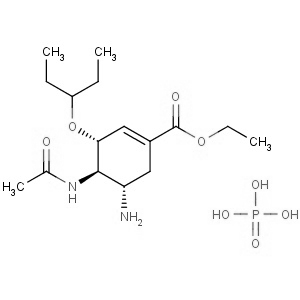
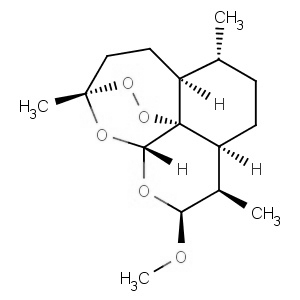
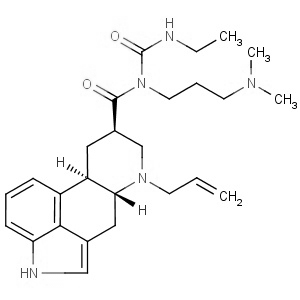
We offer from our manufacturer principals facility located in India with the SOP driven manufacturing activity carried out strictly as per ICH Q7 cGMP guidelines, and several conventional and modern techniques are used for the isolation, semi-synthesis, and purification of our products. The manufacturing processes have been validated to ensure consistent product quality.
Facilities have been inspected and approved by Regulatory authorities like US FDA, ANSM, KFDA, TGA, CDSCO, etc. They hold GMP certificates and Written Confirmation for most of our products. They have PMDA accreditation for several products