We are offering the product from our Indian manufacturer’s USFDA, Japan PMDA, Korea FDA approved site. Their facility is COFEPRIS inspected for several products. Their R&D facility is recognized by the Department of Scientific and Industrial Research (DSIR, Ministry of Science and Technology, Government of India).
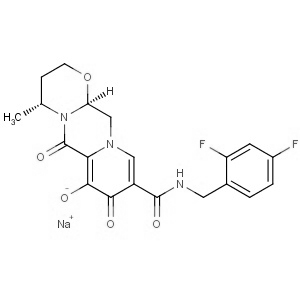
Dolutegravir
Additional information
| Product Name | Dolutegravir |
|---|---|
| CAS Registry No | 1051375-19-9 |
| Synonyms | (4R, 1-b][1, 12, 12a-hexahydro-7-hydroxy-4-methyl-6, 12aS)-N-[(2, 2':4, 3]oxazine-9-carboxamide sodium salt (1:1); Dolutegravir sodium salt; GSK 1349572A, 4, 4-Difluorophenyl)methyl]-3, 5]pyrazino[2, 6, 8, 8-dioxo-2H-pyrido[1' |
| Certification | DSIR, FDA, ISO, WHO GMP |
| Molecular Formula | C20H18F2N3O5.Na |
| Molecular Weight | 441.36 |
| MSDS Description | Available on request |
| Availability | For Research and Development Purpose |